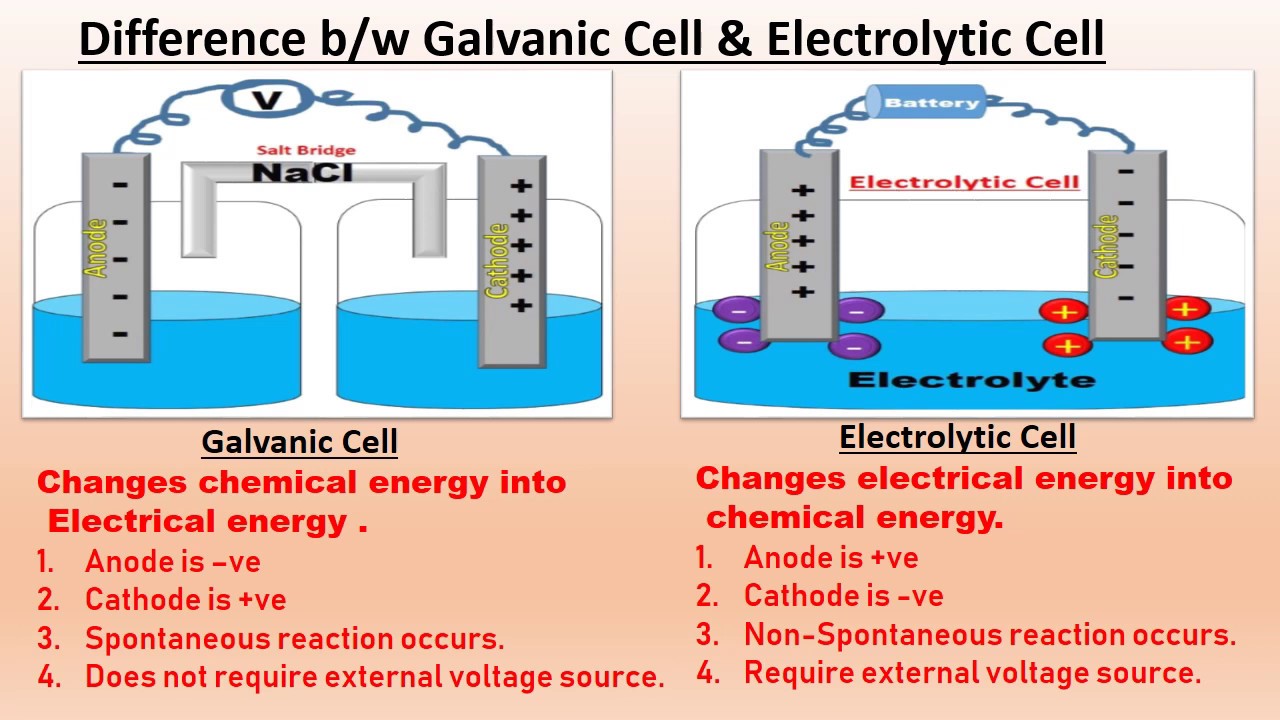
What Is The Difference Between Electrochemical And Electrolytic Cell . learn how these two types of cells operate on different principles and for different purposes. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. Find out the difference between. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams.
from www.aiophotoz.com
Find out the difference between. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn how these two types of cells operate on different principles and for different purposes.
What Is The Similarities And Differences Between Galvanic Cell And
What Is The Difference Between Electrochemical And Electrolytic Cell learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. Find out the difference between. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn how these two types of cells operate on different principles and for different purposes. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams.
From www.aiophotoz.com
What Is The Similarities And Differences Between Galvanic Cell And What Is The Difference Between Electrochemical And Electrolytic Cell Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. In an electrolytic cell, an electric current is passed through a solution to. What Is The Difference Between Electrochemical And Electrolytic Cell.
From dxofhflfn.blob.core.windows.net
Types Of Electrochemical Cells at Regina Manning blog What Is The Difference Between Electrochemical And Electrolytic Cell learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. Find out the difference between. learn the difference between galvanic and electrolytic. What Is The Difference Between Electrochemical And Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog What Is The Difference Between Electrochemical And Electrolytic Cell learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. Find out the difference between. learn how. What Is The Difference Between Electrochemical And Electrolytic Cell.
From overallscience.com
Differences between electrolytic cell and voltaic cell Overall Science What Is The Difference Between Electrochemical And Electrolytic Cell Find out the difference between. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. learn how these two types of cells operate on different principles and for different purposes. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell What Is The Difference Between Electrochemical And Electrolytic Cell Find out the difference between. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn the. What Is The Difference Between Electrochemical And Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell What Is The Difference Between Electrochemical And Electrolytic Cell learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. Find out the difference between. learn how these two types of cells operate on different principles and for different purposes. learn the points of difference between. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.artofit.org
Electrochemical cell Artofit What Is The Difference Between Electrochemical And Electrolytic Cell learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. Find out the difference between. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. Electrochemical cells. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells What Is The Difference Between Electrochemical And Electrolytic Cell learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. Electrochemical cells and electrolytic cells. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.transtutors.com
(Solved) What is the difference between a voltaic cell and an What Is The Difference Between Electrochemical And Electrolytic Cell learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. Find out the difference between. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in.. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co What Is The Difference Between Electrochemical And Electrolytic Cell learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. Electrochemical cells. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is The Difference Between Electrochemical And Electrolytic Cell learn how these two types of cells operate on different principles and for different purposes. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the points of difference between electrochemical cells and electrolytic cells with examples. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube What Is The Difference Between Electrochemical And Electrolytic Cell Find out the difference between. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. In an electrolytic. What Is The Difference Between Electrochemical And Electrolytic Cell.
From chem2u.blogspot.com
chem2U Elelctrolytic Cell and Chemical Cells What Is The Difference Between Electrochemical And Electrolytic Cell In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. Find out the difference between. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn the difference between galvanic and electrolytic cells,. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and What Is The Difference Between Electrochemical And Electrolytic Cell learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn how these two types of cells operate on different principles and for different purposes. learn how to build and use electrochemical cells, which are devices that convert redox. What Is The Difference Between Electrochemical And Electrolytic Cell.
From www.echemi.com
What is the difference between electroplating and electrolysis? ECHEMI What Is The Difference Between Electrochemical And Electrolytic Cell learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. In an electrolytic cell, an electric current is passed through a solution to induce a chemical change. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn how to. What Is The Difference Between Electrochemical And Electrolytic Cell.
From learningschoolletopisaln.z22.web.core.windows.net
Types Of Electrochemical Cells Pdf What Is The Difference Between Electrochemical And Electrolytic Cell Find out the difference between. learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn the points of difference between electrochemical cells and electrolytic cells with examples and diagrams.. What Is The Difference Between Electrochemical And Electrolytic Cell.
From llivetolearn.blogspot.com
Live to Learn, Learn to Live Electrochemical cells, Difference between What Is The Difference Between Electrochemical And Electrolytic Cell Find out the difference between. learn the difference between electrolytic cell and electrochemical cell, two types of cells that convert energy in. learn how to build and use electrochemical cells, which are devices that convert redox reactions into electrical energy. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn how these two. What Is The Difference Between Electrochemical And Electrolytic Cell.
From in.pinterest.com
What is the Difference Between Voltaic Cell and Electrolytic Cell What Is The Difference Between Electrochemical And Electrolytic Cell learn the difference between galvanic and electrolytic cells, and how they are used to drive or reverse spontaneous redox reactions. Find out the difference between. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to. learn how these two types of cells operate on different principles and for different purposes. learn the points of. What Is The Difference Between Electrochemical And Electrolytic Cell.